Our Hong Kong Foundation Launches Policy Research Report “Develop Hong Kong into Asia’s Leading Clinical Innovation Hub”
Our Hong Kong Foundation Launches Policy Research Report
“Develop Hong Kong into Asia’s Leading Clinical Innovation Hub”
(30 November 2023, Hong Kong) Our Hong Kong Foundation (OHKF), in strategic partnership with Hong Kong Science and Technology Parks Corporation (HKSTP), today released a policy advocacy report entitled Developing Hong Kong into Asia’s Leading Clinical Innovation Hub. Clinical research supports biotechnology as a strategic pillar of Hong Kong's Innovation and Technology (I&T) development. Clinical trial in particular is vital to bridging the gap between Hong Kong’s academic excellence and drug commercialisation, attracting biotechnology companies to Hong Kong as home to their new drugs’ development base and ultimately benefitting patients with the latest drugs from clinical breakthroughs. Experience of the UK National Institute for Health and Care Research (NIHR) shows that a £1 clinical trials investment can generate £7.6 in economic benefits underscoring its economic potential.
With two universities ranked top 50 globally in medicine and top 100 in pharmacy and pharmacology, plus well-recognised, world-class healthcare services and trial quality, Hong Kong has all the building blocks to become a global hub for biotechnology. In the Hong Kong Innovation and Technology Development Blueprint released last year, the Government has identified life and health sciences as a key area for development; with biotechnology recognised as an evolving industry of strategic importance in China's 14th Five-Year Plan.
Globally, the increasing need for biotechnology has continued to drive clinical trials’ development, with a predicted annualised expansion of 6% over the next decade. But while, Asia is expected to outperform clinical trials conducted in Hong Kong has seen a 22% decline between 2015 and 2021, in stark contrast to the average increase of 48% in major economies and the exponential growth of 285% in mainland China.
To identify the reasons behind Hong Kong's lagging competitive position, this study combines in-depth stakeholder interviews with an HKSTP expert survey, collectively involving more than 250 stakeholders. The report revealed three core issues that hinder Hong Kong’s competitiveness in clinical innovation.
The first issue is the shortage of clinical investigators and research support staff, with 70% of survey respondents finding the number of investigators insufficient. Overburdened hospital clinicians have little incentive to participate in clinical research in the absence of administrative support. Secondly, Hong Kong suffers from an unnecessarily long and duplicative trial approval and contract agreement timeframe relative to neighbouring economies, with 90% of respondents reporting a Clinical Trial Certificate approval process that takes more than three months for phase 1 first-in-human studies, driving pharmaceutical companies away due to commercial concerns. Thirdly, the absence of regulatory ability to conduct primary review on clinical trial results for drug registration gives companies limited incentive to put clinical trials in Hong Kong, as they first need to be recognised overseas before going through a secondary review in town based on foreign approvals.
The report proposes policy recommendations aim to transform Hong Kong into the leading clinical innovation hub in Asia, through attracting top-tier biotechnology firms, fostering talent development, and strengthening Hong Kong's role as a bridge between China and the West. The study proposes 5 policy initiatives, such as the establishment of a Clinical Research Institute to facilitate policy implementation and the creation of Hong Kong's own primary drug review authority.
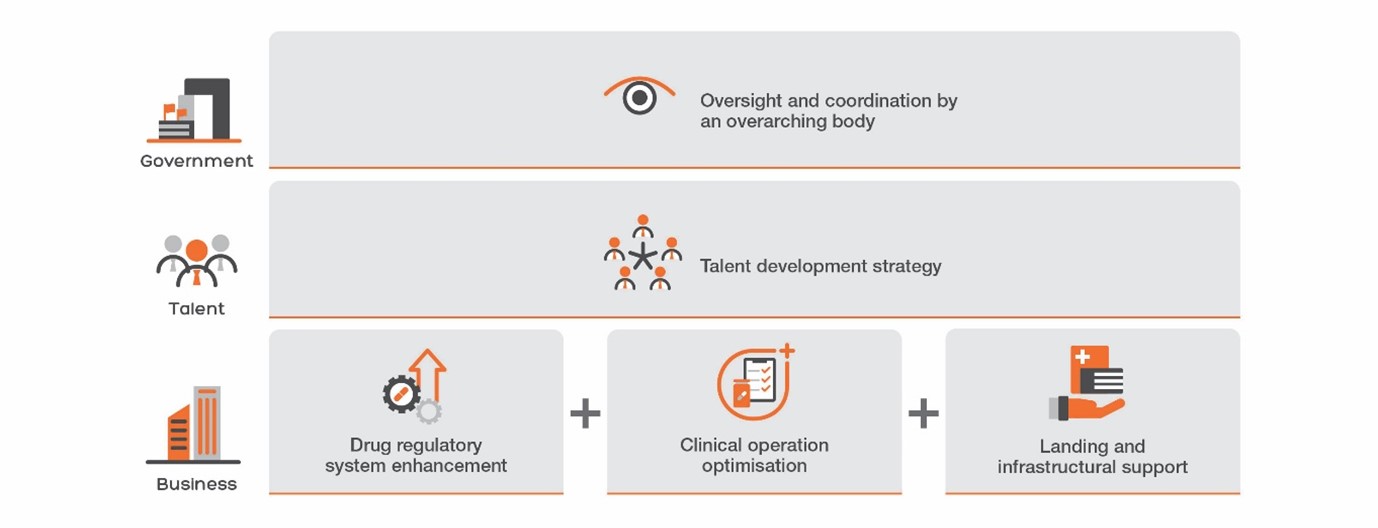
Recommendation 1. Institutionalising Strategic Facilitation by the Health Bureau. Referencing NIHR’s practices in the UK, a Clinical Research Institute (CRI) should be set up under the Health Bureau to foster concerted efforts between universities, the Hospital Authority (HA), and the private sector, expanding the scope of the current Research Office, providing crucial manpower and resources to advance clinical innovation in Hong Kong.
The CRI should perform three key functions, including to:
- act as a one-stop service centre for pharmaceutical companies, hospitals, and other agencies by providing centralised research support services, empowering non-teaching hospitals in areas such as investigator matching, site management, legal and administrative support, and laboratory services;
- bring together an international network of investigators, hence promote research collaboration and exchange. The Greater Bay Area International Clinical Trial Institute proposed in the 2023 Policy Address could broaden the network’s geographical reach. The CRI can also act as a funding intermediary and a mentorship platform for younger specialists; and
- groom clinical research talents through setting up an academy to offer professional and certified training, particularly for clinical research coordinators.
Recommendation 2. Building a Talent Development Strategy for Clinical Research Professionals. To promote and empower clinical research, the Government needs to establish career pathways for investigators, research coordinators, research nurses, and other support staff. Cognizant of the current manpower challenges in the Hospital Authority (HA), the talent strategy should focus on both nurturing young talents and maximizing the potential of existing personnel, including:
- promotions should consider clinical research achievements. Clinicians' overtime efforts should be compensated and flexible arrangements should be in place to utilise unused sponsor fees for research development;
- innovative models for balancing clinical duties and research should be implemented, such as the introduction of clinician-scientist positions and expansion of clinical research fellowships, allowing clinicians to allocate their work between clinical responsibilities and research efforts;
- additional professional training opportunities should be offered to systematically cultivate clinical research specialists. More research components should be included in tertiary education and specialist training.
Recommendation 3. Positioning Hong Kong as the “Super-connector” of Drug Development. This report calls for the Government to set up a primary review authority for new drug registrations, similar to the U.S. Food and Drug Administration (FDA), European Medicines Agency (EMA), and China’s National Medical Products Administration (NMPA). The Government has proposed to set up a preparatory office for the Hong Kong Centre for Medical Products Regulation (CMPR) in the 2023 Policy Address and has successfully joined the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) as an observer. While this is an important policy change, more needs to be done to truly catalyse systemic changes by providing a clear regulatory pathway for drug commercialisation from bench to bedside, and to build a comprehensive talent ecosystem migrating between academia, industry, and regulatory roles. Specific actions include:
- capacity building through building a regulatory pipeline for the primary review of different kinds of innovative drugs (eventually expanding the “1+” mechanism introduced in the 2023 Policy Address), and to invite global drug regulatory experts both as advisors and full-time staff;
- upon attaining primary review, seek full membership of the ICH and join multilateral agreements on drug approval
- collaborations with the NMPA through mutual abridged review for drug registration and recognition of trial approvals
- other cross-border initiatives such as cross-border research funding and easing the flow of biological samples and biochemical materials.
Although achieving the intended vision can take up to 8 years, it is envisaged that the abridged review can be achieved by 2026.
Recommendation 4. Accelerating Clinical Trials through Start-up and Capability Improvement. Hong Kong's attractiveness to businesses would be enhanced by eliminating duplicative regulatory and start-up processes. Some of these policies include:
- developing a mechanism to promote mutual recognition of ethics committees (ECs) approvals;
- granting preliminary approvals for low-risk studies upon EC approval through a Clinical Trial Notification (CTN) scheme, following the practices of Australia, Singapore, and Taiwan;
- optimizing the HA’s patient database to meet international standards and facilitate targeted access similar to the Korea National Enterprise for Clinical Trials (KoNECT).
Recommendation 5. Addressing Infrastructural and Skill Gaps to Foster Clinical Trial Activities. Critical infrastructure and expertise, in the form of central laboratories and pilot manufacturing plants, will supplement the clinical research ecosystem, thereby making Hong Kong more attractive as a clinical innovation hub. Leveraging on the New Industrialisation Acceleration Scheme announced in the 2023 Policy Address, the Government can consider targeting Contract Development and Manufacturing Organisations (CDMOs) to harness their product innovation expertise and promote talent development with the appropriate tax and land incentives.
Clinical research has significant potential for driving Hong Kong's future growth. However, several policy gaps have been identified from the perspectives of the government, talent, and businesses. As regional counterparts actively pursue opportunities in biotechnology, the Government should actively support collaborative efforts to enhance Hong Kong's clinical research capacity, attractiveness, and global connectivity, with the ultimate goal of becoming Asia's leading clinical innovation hub.
Guest of Honour, China Food and Drug Administration Former Chief and China Center for International Economic Exchanges Executive Vice Chairman Mr Bl Jingquan addressed the audience through a video speech. He indicated biomedicine has become an important symbol of China's efforts to build an innovative country. He said, “Hong Kong should further leverage its international strengths by enhancing its co-operation with large multinational companies. To improve Hong Kong's regulatory system of drugs, make good use of the opportunities arising from the development of the GBA, and to co-ordinating cross-boundary multi-centre clinical trials and strive to develop Hong Kong into an international centre for biomedical innovation.”
In his address, OHKF Governor Dr Silas Yang said, “Clinical innovation is key to Hong Kong’s future due to multiple reasons. Firstly, it is the bridge between Hong Kong’s academic and research excellence and the commercialisation of life-changing drugs and medical therapies. The recent Policy Address has taken major steps in the right direction, including a preparatory office for the Hong Kong Centre for Medical Products Regulation (CMPR) and the observer status ascension to the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH).”
OHKF President Dr Jane Lee said, “Hong Kong can leverage its world-renowned research institutions to build a clinical innovation hub that drives economic growth, serves as a gateway for innovative drugs, and ultimately improves drug access and patient care. To achieve all this, the Government needs to introduce comprehensive policies covering capacity building, international connectivity, and talent development.”
Moderated by Dr Stephen WONG, Head, Chief Executive's Policy Unit of the HKSAR Government, the panel of scholars and experts including Mr Raj BHATTI, Vice President and General Manager, GSK Hong Kong & Macau; Professor Ivan HUNG, Chair of Infectious Diseases, The University of Hong Kong; Dr Dennis LAM, Legislative Council Member, Chairman & CEO of C-MER Eye; Dr Grace LAU, Head of Institute for Translational Research, Hong Kong Science and Technology Parks Corporation; Mr Kenny SHUI, Vice President cum Co-Head of Research, Our Hong Kong Foundation; and Dr Michael WONG, Director of Quality and Safety, Hospital Authority, exchanged their views on future prospects of clinicle trials in Hong Kong and ways to nurture physician talents with research-oriented mindset.
Full Contents of the Report: Click here