Page 15 - OHKF_Biotech_EN
P. 15
Through talented individuals as well as supportive policy and
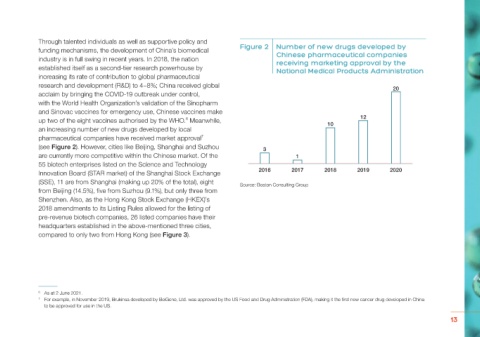
funding mechanisms, the development of China’s biomedical Figure 2 Number of new drugs developed by
Chinese pharmaceutical companies
industry is in full swing in recent years. In 2018, the nation receiving marketing approval by the
established itself as a second-tier research powerhouse by National Medical Products Administration
increasing its rate of contribution to global pharmaceutical
research and development (R&D) to 4–8%; China received global 20
上交所科創板AR Market
acclaim by bringing the COVID-19 outbreak under control, SSE ST
HKEX
with the World Health Organization’s validation of the Sinopharm 12 香港交易所
and Sinovac vaccines for emergency use, Chinese vaccines make 11 10
6
up two of the eight vaccines authorised by the WHO. Meanwhile, 10 12 8
an increasing number of new drugs developed by local
pharmaceutical companies have received market approval 4 5
7
(see Figure 2). However, cities like Beijing, Shanghai and Suzhou 3 2 3
are currently more competitive within the Chinese market. Of the 1 0 0
55 biotech enterprises listed on the Science and Technology
北京
上海
深圳
香港
蘇州
Innovation Board (STAR market) of the Shanghai Stock Exchange 2016 2017 2018 2019 2020 Shanghai Beijing Suzhou Hong Kong Shenzhen
(SSE), 11 are from Shanghai (making up 20% of the total), eight Source: Boston Consulting Group
from Beijing (14.5%), five from Suzhou (9.1%), but only three from
Shenzhen. Also, as the Hong Kong Stock Exchange (HKEX)’s
2018 amendments to its Listing Rules allowed for the listing of
pre-revenue biotech companies, 26 listed companies have their
headquarters established in the above-mentioned three cities,
compared to only two from Hong Kong (see Figure 3).
6 As at 2 June 2021.
7 For example, in November 2019, Brukinsa developed by BeiGene, Ltd. was approved by the US Food and Drug Administration (FDA), making it the first new cancer drug developed in China
to be approved for use in the US.
13